Quality testing and non-clinical safety testing of regenerative medicine products.
Staff with highly specialized skills support research and development activities for regenerative medicine products!
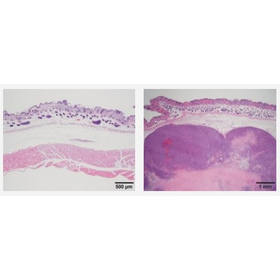
Our company conducts raw material testing, in-process control testing, and quality testing of final products for regenerative medicine products under GMP (GCPT) regulations. 【Quality Testing Items】 ■ Sterility Test (JP17 Membrane Filter Method, Direct Method) ■ Endotoxin Test (JP17 Colorimetric Method, Turbidimetric Method, Gelation Method) ■ Mycoplasma Detection Test (JP17 NAT Method using Real-time PCR) ■ Residual Testing for Antibiotics, Growth Factors, etc. (LC-MS/MS, ELISA, etc.) ■ Soft Agar Colony Formation Test, Cell Proliferation Characteristic Analysis (In Preparation) ■ Validation of Testing Methods related to the above tests In a research facility dedicated to the safety evaluation of regenerative medicine products, we conduct general toxicity tests and tumorigenicity tests using immunodeficient mice. 【Non-Clinical Testing Items】 ■ Tumorigenicity Test using Immunodeficient Mice ■ Cell Proliferation Characteristic Analysis, Soft Agar Colony Formation Test ■ General Toxicity Test ■ Safety Pharmacology Test, Efficacy Pharmacology Test ■ In Vivo Distribution Test (qPCR Method, Immunostaining) ■ Consulting for Regulatory Affairs and Applications is also available.